How Spectroscopy Is Used In Astronomy
Spectroscopy is one of the core techniques for understanding distant stars. Thanks to the fundamentals of light and how it behaves, we can collect the radiation from objects and infer many details from them.
Introduction
The study of spectral lines emitted by objects is spectroscopy. Objects emit radiation at different wavelengths and an instrument called a spectroscope analyzes it. Almost all objects will give off and absorb radiation. We know that radiation is just energy. We can not directly see these stars well enough to know much about them, that is why we have to analyze their spectra.
One of the helpful things about spectroscopy is that it is repeatable. This is very important to science. This allows other scientists to duplicate the experiments and get the same results. From there, they can work on the same topic because a hundred people working on the same thing makes faster progress.
Spectral Lines From Radiation
When you analyze spectral lines, you are studying a spectrum of light. It is the division of the radiation’s component wavelengths. This means the light has been divided up. Every object gives off multiple forms of radiation. These will be at different wavelengths. The reason we want to analyze spectra is that it will give us a lot of information about the object that originally emitted the radiation.
intensity
frequency
energy
temperature
magnetic field
When analyzing spectral lines, you will notice they are all very thin. There are differences, but they are small. The lines you get depend on what you are studying. It could be a single atom or a complex object like a star.
The tool used to analyze radiation is the spectroscope. It is one of the core instruments of spectroscopy. Its major component is a prism that splits the light into its wavelengths. There are other smaller parts that help refine the process. You usually look through an eyepiece like on a telescope.
Emission Line Spectra
Emission lines are what we see when we split the radiation into parts. Keep in mind when you do this, only the more prominent parts of the spectrum will show up. This characterizes each object, these distinct emission lines for every object. When we repeat this process, we get the same type of emission lines each time. If we analyze light from another source, then the lines would be totally different.
When looking at an object through a spectroscope you should see black lines throughout the spectrum. These black lines are actually gaps. They represent wavelengths that should be there but are not. Something happened to them so they did not show up. These lines are officially called Fraunhofer lines or absorption lines and are a key ingredient to the study of spectroscopy.
Kirchhoff’s Laws
A German physicist named Gustav Kirchhoff noticed there were similarities between types of spectra. He had a few famous laws.
An object under high heat and pressure gives off a continuous spectrum.
A gas with high heat and low pressure gives off an emission line spectrum.
An object that emits a continuous spectrum and is behind a cool gas under pressure exhibits an absorption spectrum.
Decomposing Starlight
The last couple hundred years the field of spectroscopy has developed quickly and so scientists had several ideas on how to read starlight. One of the most important things that was learned was that the lines on a spectrum corresponded to individual elements. So developing a spectrum for an object meant we could discern its composition.
This is very important because we can’t physically observe stars. So this method allows scientists to determine the makeup of faraway objects. Far away objects are commonly stars. Knowing what makes up a star tells us how it was formed and in what conditions.
When we know under what conditions something it created, we can infer age, size, and velocity. So every piece of information we gather leads to more discoveries.
Atoms and Radiation
Atoms are the basic units from which everything is created. Even at their small size radiation affects them. Atoms absorb radiation which changes the atom. Historically, the electrons in an associated atom were thought to be on precise orbits, kind of like planets.
Scientists now think that it is a very loose orbit around the nucleus of an atom. Some may even be scattered about.
Before modern times we thought everything behaved only like a wave. It made sense most of the time and was assumed to be true for a long time. Eventually, however, our technology improved, as did our instruments and theory.
There was something missing, something not quite right. The answer we now know is that light does not behave purely as a wave. Sometimes it acts like a particle too.
Electrons
Electrons are only found in atoms having a certain transfer. This goes whether they are absorbing or transmitting energy. So the radiation involved in these transactions must correlate to the difference in their separate states. They are known as photons, and are the particles we now know.
A photon is electromagnetic radiation. According to Einstein, the energy of a photon was about the same as the frequency of the radiation. This concept was later worked on in depth by Max Planck and is known as Planck’s constant.
Every element has its own signature on the spectrum.
Hydrogen
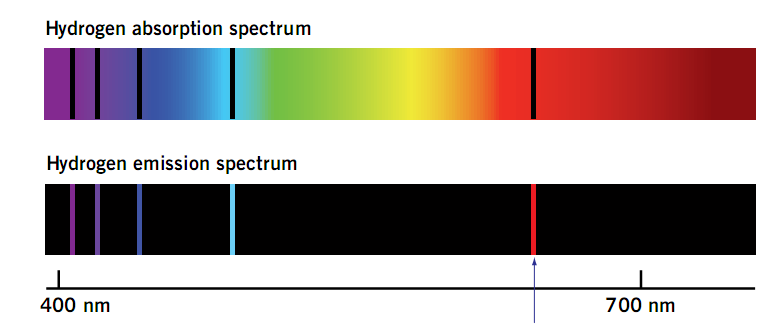
The spectrum of hydrogen covers most of the electromagnetic spectrum. When electrons absorb radiation, they then have more energy. Electrons do not keep this energy though. They will lose it. The question is how fast.
If it is quickly lost, then it will emit a single photon with the excess energy but if lost slowly it will emit two photons with the absorbed energy in both. Photons during this process can have differing amounts of energy obviously.
So each photon will be at a different wavelength. Therefore, the spectrum of an object has many lines at different frequencies and wavelengths. The photons that were released after absorbing energy were each given a different amount of radiation or energy.
Carbon And Later Elements
An atom with more protons will have a much more complicated emission spectra. The reason is that there are many more electrons that can get charged and developed into an active state. Each one could get a differing amount of radiation and so give off emissions at a different frequency.
Every different frequency will make the spectra taken from the atom look different. So you can see how different and complicated this can be quickly. Carbon and later atoms of course have larger and larger amounts of protons and electrons.
Molecular Spectra
What happens when different atoms join and make a molecule? Well, things just get interesting. Those different atoms do not stop emitting radiation. They are all doing it at the same time or just a few at once. It depends on what happens with their electrons and what particular atoms are involved.
Since a molecule is just a bunch of different atoms held together by a chemical reaction between their respective electrons, we can expect there to be many more rules about how they interact. The spectra that are emitted from various molecules is therefore a combination of those atoms that make up the molecule.
Analysis Of Spectra
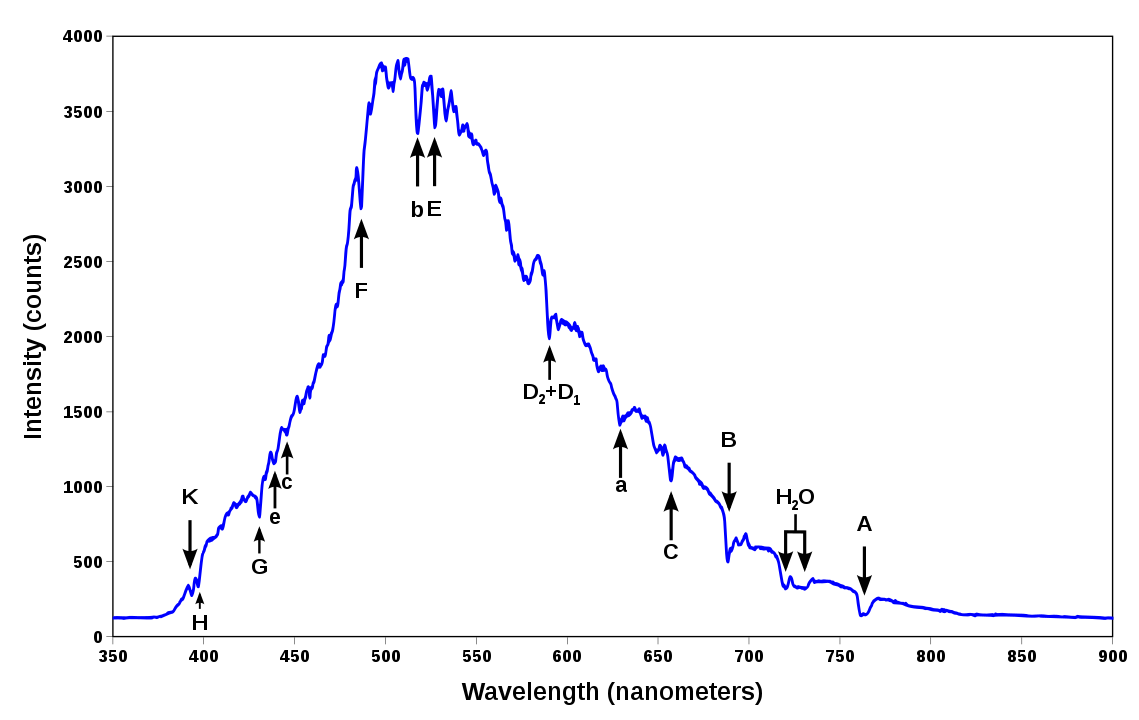
Now that you have sort of an idea of how all of this works, what is exactly is the point you may ask? The very interesting and valuable point is that you use the emission spectra to infer critical information from what you are observing. This can be a star, galaxy, black hole, or planet. Almost everything we know from space is from these studies of radiation that reach us here on Earth.
Composition of Objects
This is another example where the light tells us a lot of information. As light moves through the photosphere of a star, it absorbs the information of things it passes through. These things can be gases or dust, for example.
Temperature Of Objects
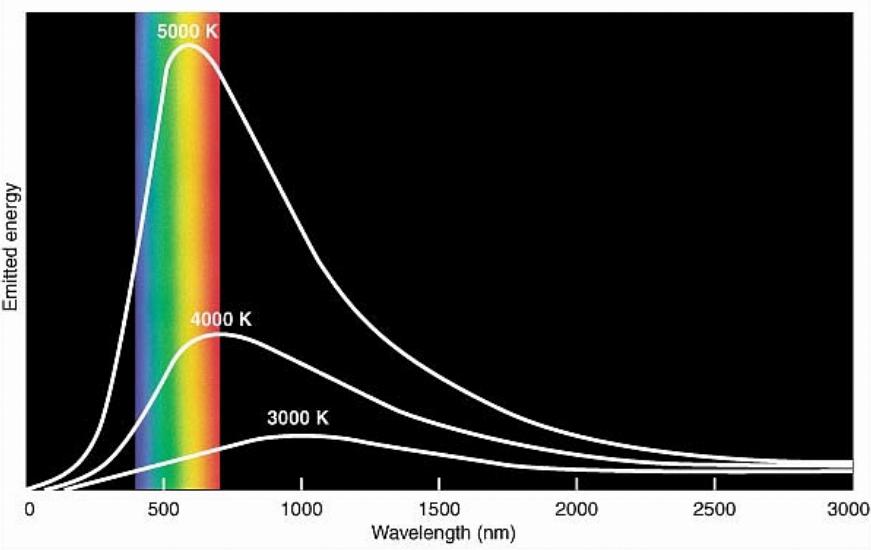
Measuring the temperature is one of the important tasks for an astronomer. Knowing the temperature alone gives us a little information but put it together with known atoms and molecules and we get a much better picture of what is going on.
Things emit light and heat because they are hot or emit some kind of heat. Different temperatures tell us approximately what the object is that emitted it. A star that is red tells us the star is on the cooler than a star that is more blue. This makes it handy in identifying objects and their temperatures.
Speed Of Objects
The shifted lines of an object can tell us its velocity. The well known Doppler effect does this. Looking at the source radiation and checking for either red-shifted or blue-shifted lines can tell us a lot. If they are blue-shifted, then the source is moving toward us. Red-shifted lines mean the source is moving away from the observer.
Width Of Spectral Lines
Even the width of these spectral lines can tell us a lot about what is going on. The width indicates the environment of the source. Most often the velocity tells a lot. A higher velocity will usually mean a higher temperature too. Magnetic fields can also broaden spectral lines. The stronger the magnetic field the more broad the line usually is.
Conclusion
In this article, we have discussed spectral lines, and why they are useful. Spectral lines are a field of study, called spectroscopy. This is a very useful field because all light or radiation gives clues about where it came from. These clues include temperature, velocity, and composition of the original source of the light.
Thanks For Reading
I hope you enjoyed this article. I appreciate you reading it. Astronomy and its subtopics are a wonderful topic and I enjoy writing about it.
If you would like to read more Astronomy articles, here are some options.
https://sciencebyjason.com/astronomy-for-beginners-radiation.html
and
https://sciencebyjason.com/orbital-motion-in-the-solar-system.html